Lyophilization Cycle
Development & Optimization

Lyophilization Center of Excellence
Optimizing lyophilization cycle times and improving stability for complex injectables is a critical component of parenteral product development. Operating at the forefront of lyophilization optimization, we maintain an industry-leading Lyophilization Center of Excellence, co-located with our facilities in Bloomington, Indiana, USA, and Halle/Westfalen, Germany. We offer considerable expertise in refining freeze-drying techniques. Our scientists and affiliated staff can work with you to make process modifications and adjust formulas to capitalize on the full potential of your lyophilized products.

Advantages of Our Approach
Our “quality-by-design,” or “design space,” approach to freeze-drying cycle development has significant advantages over more traditional trial-and-error approaches. It reduces both the amount of active pharmaceutical ingredient and the time required for development, includes edges of failure to help ensure pharmaceutically acceptable product, facilitates handling of deviations and provides experimentally determined residual moisture specification.
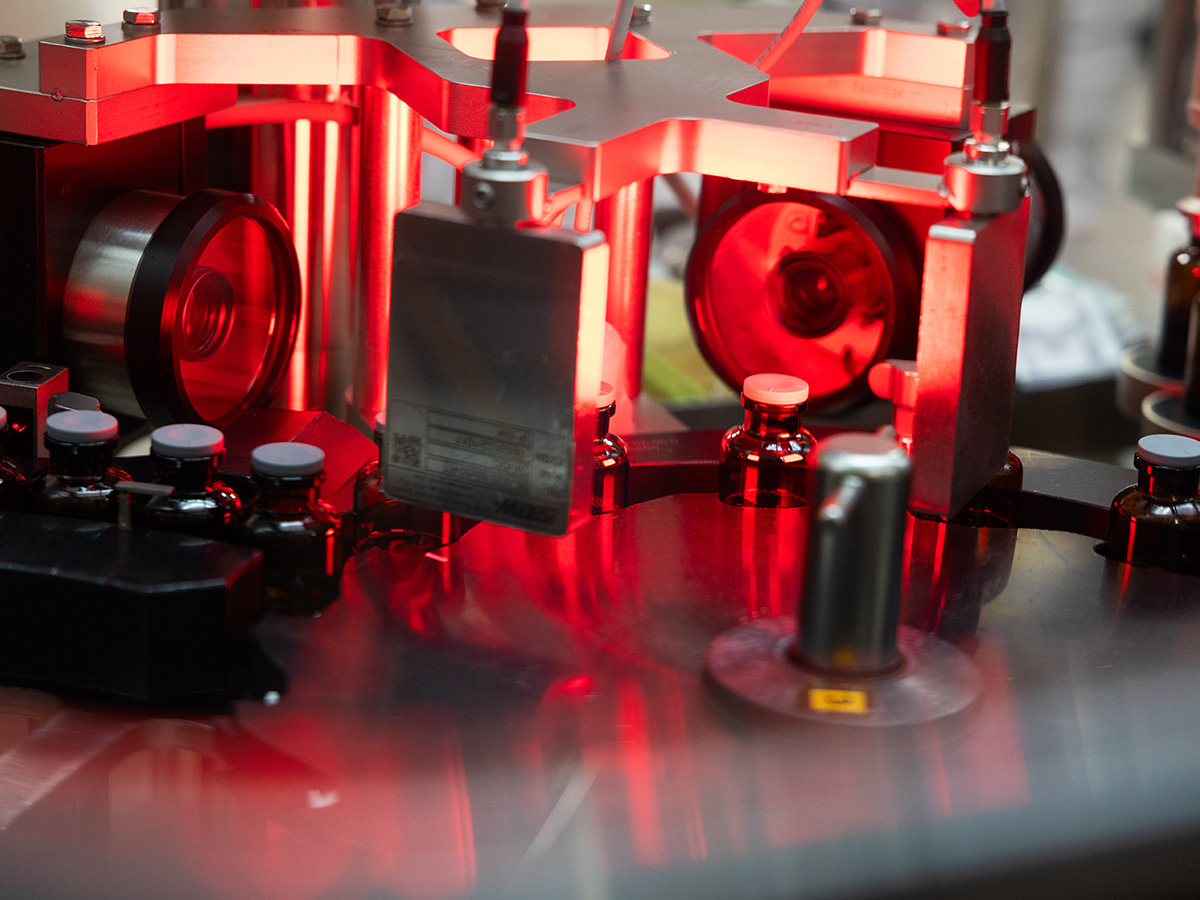
Cutting-Edge Capabilities, Customized Service
We use state-of-the-art instrumentation for the detection of aggregation and other physical stability issues and the latest analytical methodologies for protein biophysical characterization and measurement of protein aggregates. To minimize handling and microbial exposure during production, we offer robotic loading and unloading of lyophilizers.
Ultimately, our approach to lyophilization helps to identify the shortest possible and most robust lyophilization cycle in order to reduce manufacturing cost. And as always, we offer customized service, whether you have not yet developed a lyophilization cycle for your product’s formulation or recognize a need to shorten or enhance it.
Contact Us
Learn more about our lyophilization cycle development and optimization services.
