The Power of Two.
The Ease of One.
An ADC Manufacturing Alliance

Delivering What Matters, Together
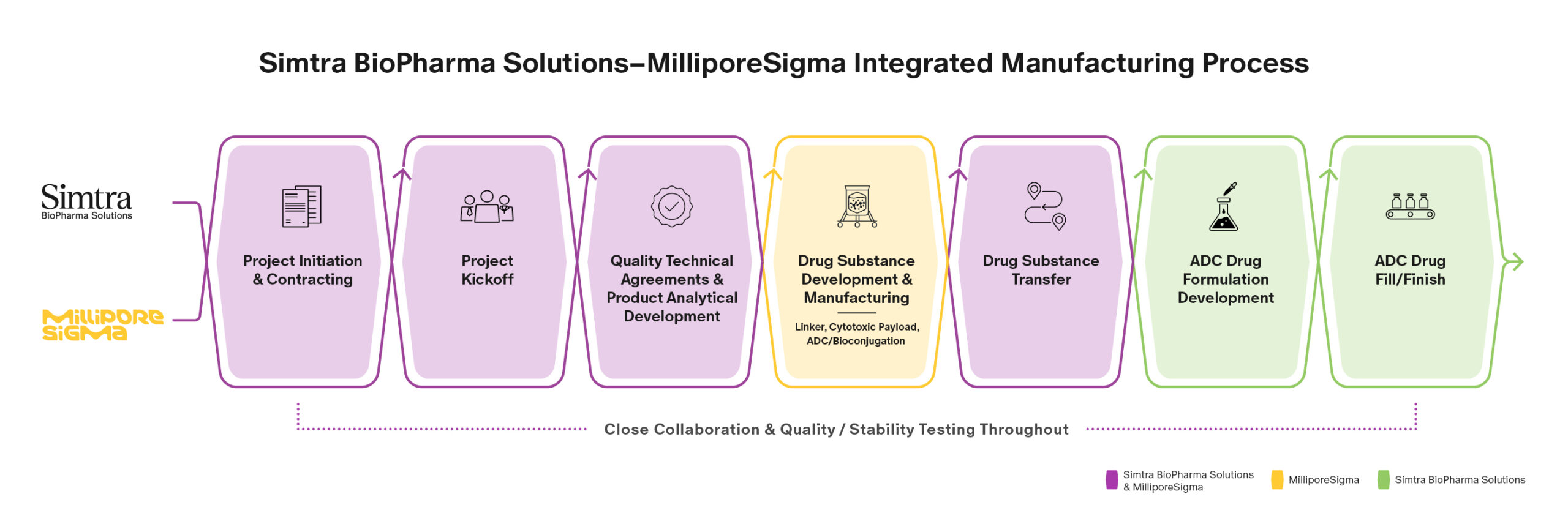
With the development and clinical use of antibody-drug conjugates (ADCs) currently gaining significant momentum, the demand for contract development and manufacturing services continues to increase. In response, Simtra BioPharma Solutions (Simtra) and MilliporeSigma have forged a strategic alliance to streamline and accelerate the delivery of these targeted therapies and other bioconjugates to patients.
Two Experts,
One Streamlined Solution
As two industry-leading contract development and manufacturing organizations (CDMOs), both with over 10 years of experience, we are united by a shared goal to more efficiently deliver ADCs to the market. Committed to excellence and timely product delivery, Simtra and MilliporeSigma will align their respective expert capabilities and collaborate on program management to provide a quality-focused turnkey solution that facilitates the supply of these complex drugs.
Strong Partners, Multiple Benefits
Coordination of the expertise and experience of Simtra and MilliporeSigma yields numerous benefits that enable the allied teams to meet unique product requirements and successfully reduce complexity, shorten timelines and create operational synergies.
-
Simplified supply chain:
- Fewer CDMOs to screen for selection
- Designated project management
- Synchronized fill slot scheduling
-
Harmonized processes:
- Separate but concurrent proposals, coordinated contracting and quality agreements
- Seamless material transfer
- Integrated formulation development
-

Standardized practices:
- Seamless testing and material onboarding
- Global logistics handling
- Transparency on entire drug substance–drug product manufacturing process

Simtra BioPharma Solutions Capabilities
Simtra BioPharma Solutions is an independent CDMO established with the acquisition of Baxter’s BioPharma Solutions business.
- 65+ years of sterile injectable manufacturing experience
- 10+ years of experience in manufacturing ADCs
- Transfer of more than 60 ADC programs for 15 distinct clients, including 6 commercially approved products
- World-class cGMP sterile fill/finish services
- Deep scientific and technical expertise
- Uniquely collaborative approach to support customers’ strategic objectives
- Quality-driven, long-tenured experts
- Tailored clinical or commercial manufacturing solutions
- Simtra-manufactured products distributed in 120 countries around the world

MilliporeSigma Capabilities
MilliporeSigma is the Life Science business of Merck KGaA, Darmstadt, Germany, in the U.S. and Canada.
- 15+ years of leading expertise in conjugation of drug linkers to mAbs
- First commercially approved ADC manufacturer in North America
- Track record of supporting over 70 INDs and manufacturing more than 30 commercial GMP batches
- Facilities specialized for handling highly potent compounds
- Integrated linker and payload CDMO services, including innovative ADCore payload intermediates
- Ongoing expansion in ADC capabilities and capacity
Get in Touch
Let us tell you more about how our allied teams can help you achieve your ADC manufacturing objectives.
