Services
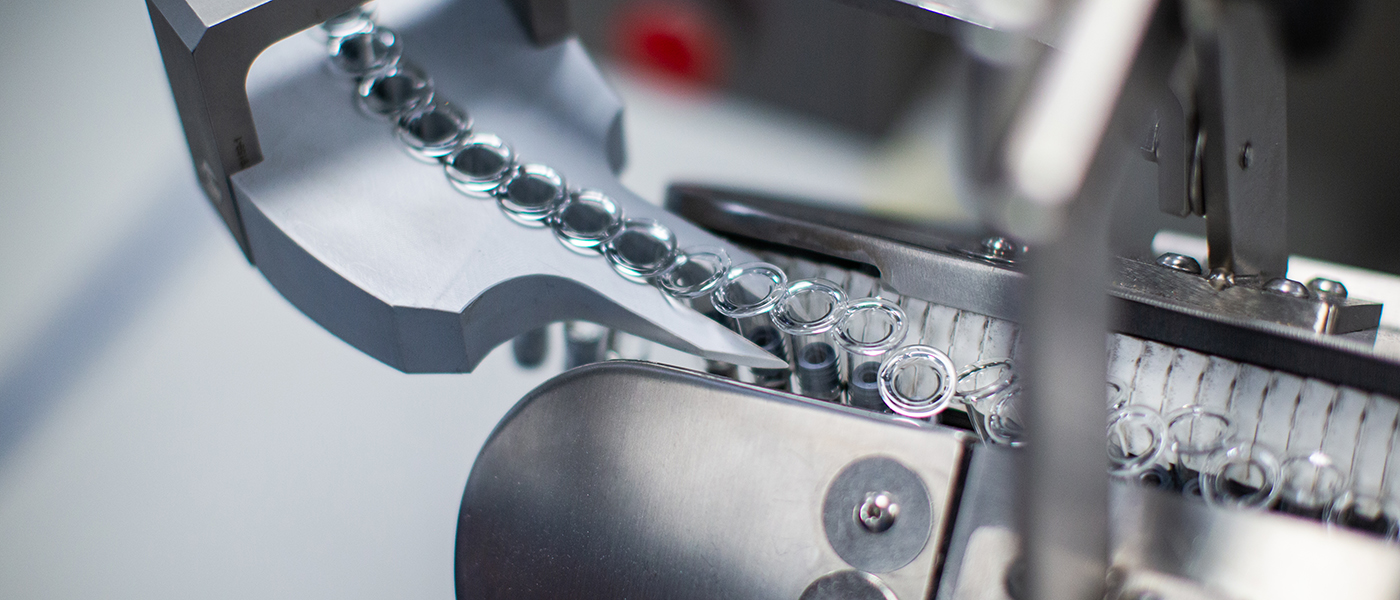
Services from Clinical to Commercial
Manufacturing parenteral products presents numerous high-stakes challenges: formulation and reformulation issues, delivery system conversion, upscaling from clinical to commercial production, market fluctuations in demand, new indications, emerging markets, risk mitigation, patent expiry and more. As your development and manufacturing partner, we are committed to collaborating with you to overcome such challenges, utilizing our clinical-to-commercial range of services — customized to your explicit needs — with the goal of maximizing product quality and speed to market.
Explore Our Services
-

Formulation Development
Leveraging our scientific and technical expertise in sterile-injectable manufacturing partnerships.
-

Lyophilization Cycle Development and Optimization
Using advanced technology to assist clients with lyophilization cycle development and optimization.
-

Rapid Technology Transfer
Ensuring rapid technology transfer through strong partnership.
-
Fill/Finish Operations
Precisely coordinating our fill/finish operations to ensure quality and timely delivery of product.
-
Specialized Containment
Utilizing restricted access barrier system technologies to ensure safety and regulatory compliance.
-

Analytical Services
Offering robust analytical capabilities to ensure stringent quality assurance and precise testing.
-
Global Regulatory Support
Providing expert guidance to help clients execute regulatory strategies and prepare filings.
Contact Us
Learn more about how our services can help you optimize your product’s potential.
