From Lab
Through Launch
Your Partner at Every Step

Why Choose Simtra?
As the need for sterile injectables grows, full-service contracted support can enable you to meet increasing demands for new product development, manufacturing and commercialization. As a leading contract development and manufacturing organization (CDMO) with 65+ years of experience, Simtra BioPharma Solutions provides customized end-to-end services that can help get you to market faster.
Our Development and Pre-Commercial Services Continuum
Completed prior to commercial manufacturing, our development and pre-commercial services (DPCS) continuum starts with product formulation development, analytical methods development and manufacturability assessment, and continues through clinical production and process validation.
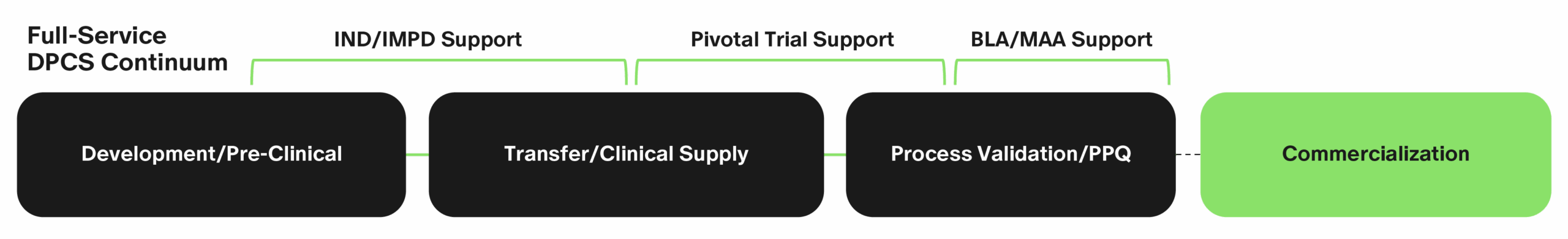
Unlock Efficiencies With Simtra
Our development and pre-commercial services approach minimizes risk, avoids disruptive handoffs, safeguards product knowledge, accelerates timelines and ensures readiness for regulatory approval and commercial production. Our quality-driven, full-service approach is made for this.
-
Product Design With the End in Mind
-
The Right Partner
-
Integrated Expertise Under One Roof
-
Proven Methodologies, Expertly Implemented
-

Commitment to Excellence

Product Design With the End in Mind
From the outset, we design products for high-quality manufacturing to maximize yields, minimize loss and facilitate scalability, saving time and money.
- Work towards a Quality Target Product Profile to ensure safety, effectiveness and regulatory compliance
- Conduct manufacturability assessments
- Ensure acceptable routine manufacturing processing ranges

The Right Partner
We are a right-sized organization — agile and adaptable — for true partnership.
- Work closely with stakeholders to tailor solutions, engaging a dedicated account executive, a seasoned project manager and multidisciplinary experts
- Guarantee project team capacity before commitment
- Meet with clients regularly to ensure transparency, resulting in alignment on data results and next steps, collaboration to overcome challenges and fully realized feasible expectations
- Maintain strong client partnerships at our facilities in Bloomington, Indiana, U.S.A., and Halle/Westfalen, Germany
Integrated Expertise Under One Roof
Our integrated approach to workflow, cross-functional expertise, continuity of project management and extensive product knowledge streamline our development and pre-commercial services continuum and accelerate readiness for commercialization.
Seamless Functional Integration
- Continuous, transparent workflow that drives consistent quality, reduces complexity and risk, expedites timelines and contains costs
- Synergy between scientific, tech transfer, development and manufacturing teams
- Continuity of project manager from start to finish
- Equipment configured for ease of scale-up
End-to-End Expertise
- Development team of 20+ scientists; average of 15 years’ experience
- Scientists engaged with university and industry consortia and standard-setting organizations, and published in peer-reviewed journals
- PMI-certified project managers; many with scientific or functional expertise
- CDMO experience with cytotoxics (including ADCs), highly potent compounds, small-molecule drugs and biologics (including vaccines)
- 100+ products manufactured annually
- Harmonized technical transfer services across both sites

Proven Methodologies, Expertly Implemented
We continuously invest in industry-leading technology and leverage proven processes so that we can develop efficient, cost-effective and innovative solutions for even the most complex products.
- Proven ability to solve a wide range of formulation development issues
- Successful entry into clinical trials for all Simtra-developed formulations
- 20+ years’ experience in lyophilization development/optimization
- Industry-leading Lyophilization Center of Excellence staffed by experts using advanced technologies
- “Design space” lyophilization optimization approach
- Robust analytical capabilities and state-of-the-art instrumentation
Commitment to Excellence
Our relentless focus on quality and performance means that we seek to exceed industry standards with every project.
- Strong global regulatory audit and inspection history in both Bloomington, Indiana, U.S.A., and Halle/Westfalen, Germany
- Quality focus apparent in sterility record
- Diligent focus on continuous process improvement
- Extensive global regulatory experience that helps minimize compliance risks and production delays
- SafeBridge certification in Halle/Westfalen, Germany
Your Dedicated Partner
Simtra BioPharma Solutions is your dedicated partner at every step, from development and pre-commercial services through commercial production. We are made for this.
If you would like to discuss how Simtra can help you achieve your commercialization objectives, we invite you to initiate a project with us.
